10 terms mzapien13 Preview Functional Groups in Chemistry 10 terms Ecollins244 Preview quiz 2 75 terms Rubi_Jimenez Preview Chem pKas for functional groups
Which of the following will be dehydrated most readily in alkaline med
Nov 21, 2023Acid-catalyzed dehydration of alcohols occurs when an acid puts an extra hydrogen atom on the alcohol. This changes the OH alcohol group into an H 2 O, or water, group attached to the organic
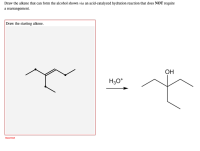
Source Image: bartleby.com
Download Image
login Login how_to_reg Request Instructor Account hub Instructor Commons Search Search this book Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more Periodic Table Physics Constants Scientific Calculator Reference expand_more Reference & Cite Tools expand_more Help expand_more Get Help Feedback

Source Image: pubs.acs.org
Download Image
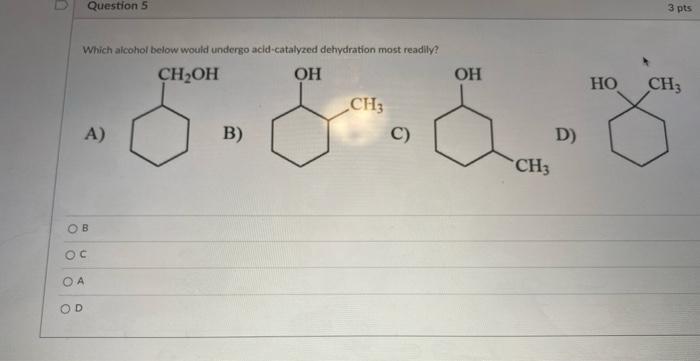
Solved Question 5 3 pts Which alcohol below would undergo | Chegg.com Phosphoric(V) acid tends to be used in place of sulphuric acid because it is safer and produces a less messy reaction. Phosphoric(V) acid isn’t a strong oxidising agent. The dehydration of more complicated alcohols. You have to be wary with more complicated alcohols in case there is the possibility of more than one alkene being formed.
Source Image: numerade.com
Download Image
Which Alcohol Below Would Undergo Acid-Catalyzed Dehydration Most Readily
Phosphoric(V) acid tends to be used in place of sulphuric acid because it is safer and produces a less messy reaction. Phosphoric(V) acid isn’t a strong oxidising agent. The dehydration of more complicated alcohols. You have to be wary with more complicated alcohols in case there is the possibility of more than one alkene being formed. What does acid-catalyzed dehydration synthesize? An alkene from an alcohol. What is an elimination reaction? A type of organic reaction in which two substituents are removed resulting in the formation of a pi bond. What happens in most organic eliminations? A proton and a leaving are removed to make the alkene.
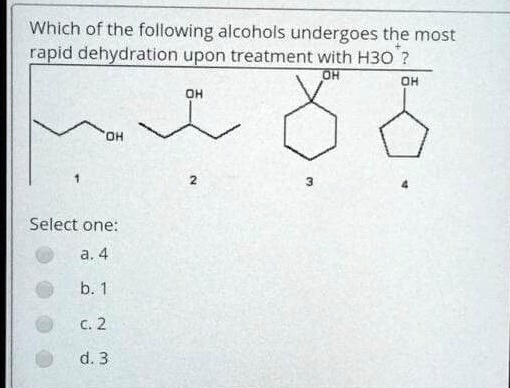
SOLVED: Which of the following alcohols undergoes the most rapid dehydration upon treatment with H3O Select one: c2 d.3
Jan 16, 2024Secondary alcohols can be made to react, but the conditions are severe (75% H 2 SO 4, 100 °C) and sensitive molecules don’t survive. Figure 17.7 MECHANISM Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate. Acid-catalysed dehydration of which of following compounds is fastest?
Source Image: toppr.com
Download Image
SOLVED: 56. Which alcohol will undergo acid-catalyzed dehydration under the mildest conditions? (A) CH3CH2CH2CH2OH (B) CH3CH2CH(OH)CH3 (C) (CH3)2CHOH (D) CH3COH Jan 16, 2024Secondary alcohols can be made to react, but the conditions are severe (75% H 2 SO 4, 100 °C) and sensitive molecules don’t survive. Figure 17.7 MECHANISM Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate.
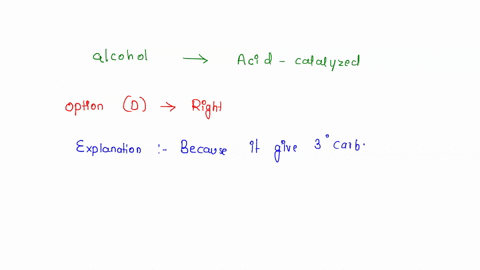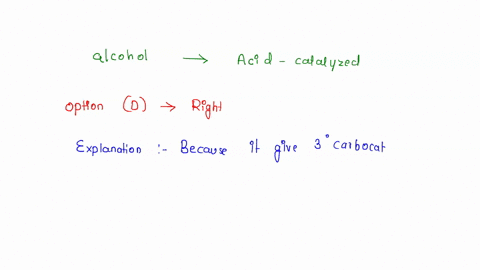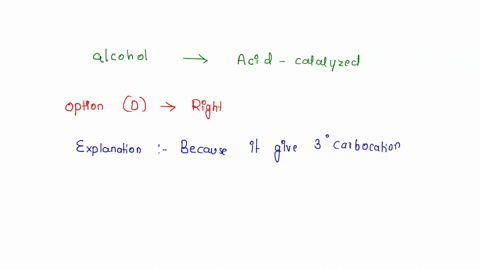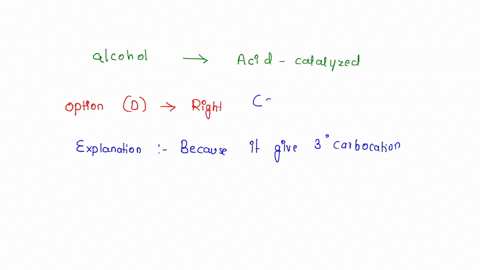
Source Image: numerade.com
Download Image
Which of the following will be dehydrated most readily in alkaline med 10 terms mzapien13 Preview Functional Groups in Chemistry 10 terms Ecollins244 Preview quiz 2 75 terms Rubi_Jimenez Preview Chem pKas for functional groups
Source Image: doubtnut.com
Download Image
Solved Question 5 3 pts Which alcohol below would undergo | Chegg.com login Login how_to_reg Request Instructor Account hub Instructor Commons Search Search this book Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more Periodic Table Physics Constants Scientific Calculator Reference expand_more Reference & Cite Tools expand_more Help expand_more Get Help Feedback
Source Image: chegg.com
Download Image
What is the mechanism for the acid catalyzed dehydration of an alcohol to form an alkene? | Homework.Study.com Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate. To circumvent the need for strong acid and allow the dehydration of secondary alcohols in a gentler way, reagents have been developed that are effective under mild, basic conditions.

Source Image: homework.study.com
Download Image
Chemistry 210 – Chapter 5 – Quiz 2 Phosphoric(V) acid tends to be used in place of sulphuric acid because it is safer and produces a less messy reaction. Phosphoric(V) acid isn’t a strong oxidising agent. The dehydration of more complicated alcohols. You have to be wary with more complicated alcohols in case there is the possibility of more than one alkene being formed.
Source Image: home.miracosta.edu
Download Image
SOLVED: Which alcohol below would undergo acid-catalyzed dehydration most readily? What does acid-catalyzed dehydration synthesize? An alkene from an alcohol. What is an elimination reaction? A type of organic reaction in which two substituents are removed resulting in the formation of a pi bond. What happens in most organic eliminations? A proton and a leaving are removed to make the alkene.

Source Image: numerade.com
Download Image
SOLVED: 56. Which alcohol will undergo acid-catalyzed dehydration under the mildest conditions? (A) CH3CH2CH2CH2OH (B) CH3CH2CH(OH)CH3 (C) (CH3)2CHOH (D) CH3COH
SOLVED: Which alcohol below would undergo acid-catalyzed dehydration most readily? Nov 21, 2023Acid-catalyzed dehydration of alcohols occurs when an acid puts an extra hydrogen atom on the alcohol. This changes the OH alcohol group into an H 2 O, or water, group attached to the organic
Solved Question 5 3 pts Which alcohol below would undergo | Chegg.com Chemistry 210 – Chapter 5 – Quiz 2 Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate. To circumvent the need for strong acid and allow the dehydration of secondary alcohols in a gentler way, reagents have been developed that are effective under mild, basic conditions.