The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Example 21.18.1 21.18. 1. In a titration of sulfuric acid against sodium hydroxide, 32.20mL 32.20 mL of 0.250M NaOH 0.250 M NaOH is required to neutralize 26.60mL 26.60 mL of H2SO4 H 2 SO 4.
Titrations: Techniques and Calculations – Carolina Knowledge Center
To calculate the pH of the solution, we need to know [H+], which is determined using exactly the same method as in the acetic acid titration in Example 17.4.2: final volume of solution = 100.0mL + 55.0mL = 155.0mL. Thus the concentrations of Hox− and ox2− are as follows: [Hox−] = 3.60 mmolHox− 155.0 mL = 2.32 ×10−2 M.

Source Image: tes.com
Download Image
There are three scenarios we will consider, using the titration of 50.0 mL of 0.100 M acetic acid with 0.200 M NaOH (Figure 7.4.1a) as an example: The pH at the beginning of the titration, before any titrant is added. The pH in the buffer region, before reaching the equivalence point. The pH at the equivalence point.

Source Image: scribd.com
Download Image
SOLVED: A 25.0-mL sample of an unknown HBr solution is titrated with 0.100 M NaOH. The equivalence point is reached upon the addition of 18.88 mL of the base. What is the The titration of 80.0 mL of an unknown concentration H* 3PO4 * solution requires 126 mL of 0.218 M (molarity) KOH solution. What is the concentration of H* 3PO4 * solution (in M)? I go through all of the calculations and get 0.343 M, but apparently this is wrong. I’m pretty sure that I’ve set it up correctly. I’m lost here. Sort by:

Source Image: about.dataclassroom.com
Download Image
The Titration Of 80.0 Ml Of An Unknown
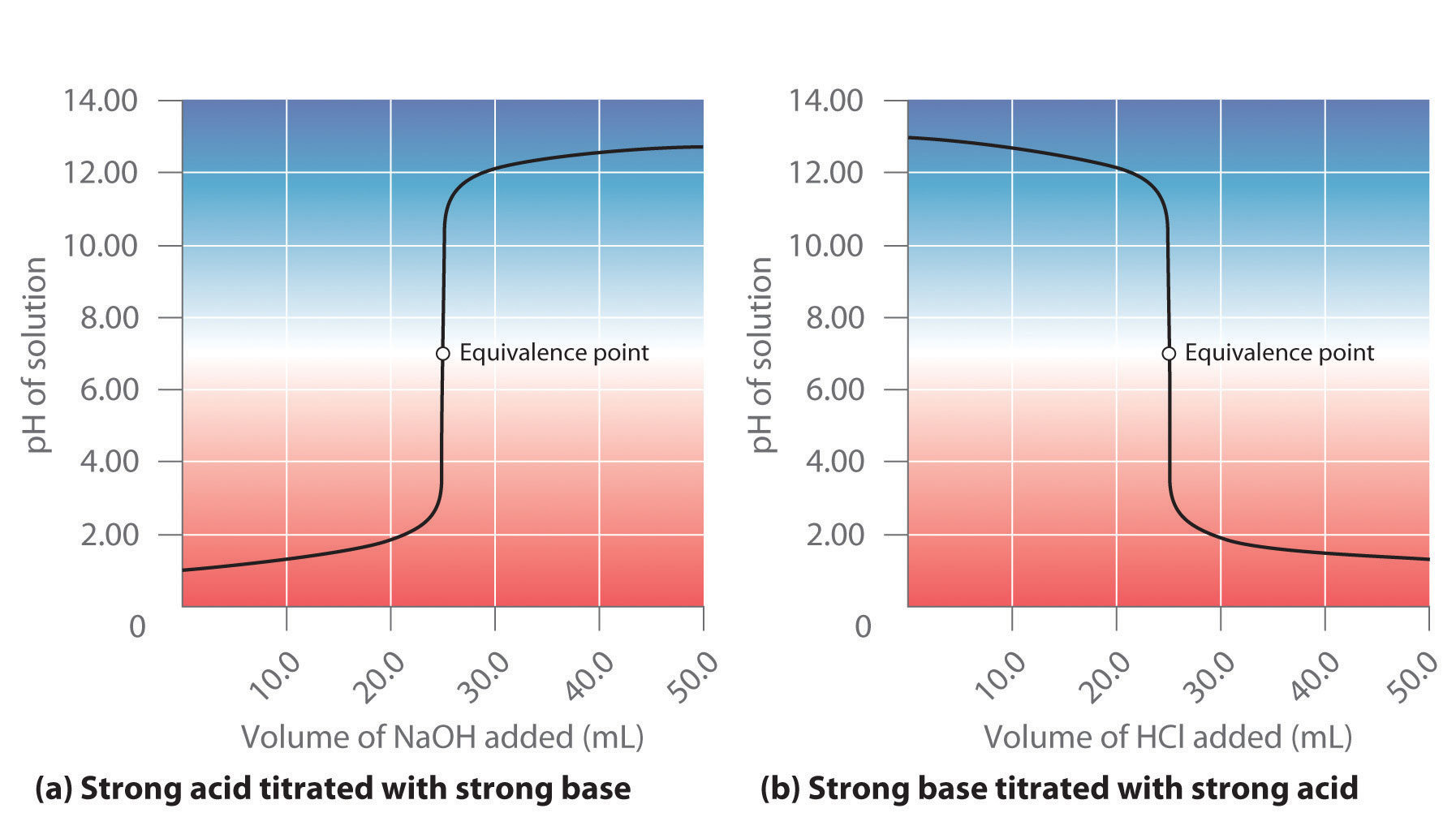
The titration of 80.0 mL of an unknown concentration H* 3PO4 * solution requires 126 mL of 0.218 M (molarity) KOH solution. What is the concentration of H* 3PO4 * solution (in M)? I go through all of the calculations and get 0.343 M, but apparently this is wrong. I’m pretty sure that I’ve set it up correctly. I’m lost here. Sort by: A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH (the titration curve is shown in Figure 14.18). Calculate the pH at these volumes of added base solution: (a) 0.00 mL (b) 12.50 mL (c) 25.00 mL (d) 37.50 mL. Solution (a) Titrant volume = 0 mL. The solution pH is due to the acid ionization of
Acid-Base Titration Lab — DataClassroom
Science Chemistry Chemistry questions and answers The titration of 80.0 mL of an unknown concentration HCl solution requires 151.9 mL of 0.42 M KOH solution to reach the equivalent point. How much of HCl was originally added to the solution? Consider the titration of a 25.0-mL sample of 0.175 M CH3NH2 with… | Channels for Pearson+

Source Image: pearson.com
Download Image
Consider the titration of a 25.0-mL sample of 0.175 M CH3NH2 with… | Channels for Pearson+ Science Chemistry Chemistry questions and answers The titration of 80.0 mL of an unknown concentration HCl solution requires 151.9 mL of 0.42 M KOH solution to reach the equivalent point. How much of HCl was originally added to the solution?

Source Image: pearson.com
Download Image
Titrations: Techniques and Calculations – Carolina Knowledge Center The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Example 21.18.1 21.18. 1. In a titration of sulfuric acid against sodium hydroxide, 32.20mL 32.20 mL of 0.250M NaOH 0.250 M NaOH is required to neutralize 26.60mL 26.60 mL of H2SO4 H 2 SO 4.

Source Image: knowledge.carolina.com
Download Image
SOLVED: A 25.0-mL sample of an unknown HBr solution is titrated with 0.100 M NaOH. The equivalence point is reached upon the addition of 18.88 mL of the base. What is the There are three scenarios we will consider, using the titration of 50.0 mL of 0.100 M acetic acid with 0.200 M NaOH (Figure 7.4.1a) as an example: The pH at the beginning of the titration, before any titrant is added. The pH in the buffer region, before reaching the equivalence point. The pH at the equivalence point.
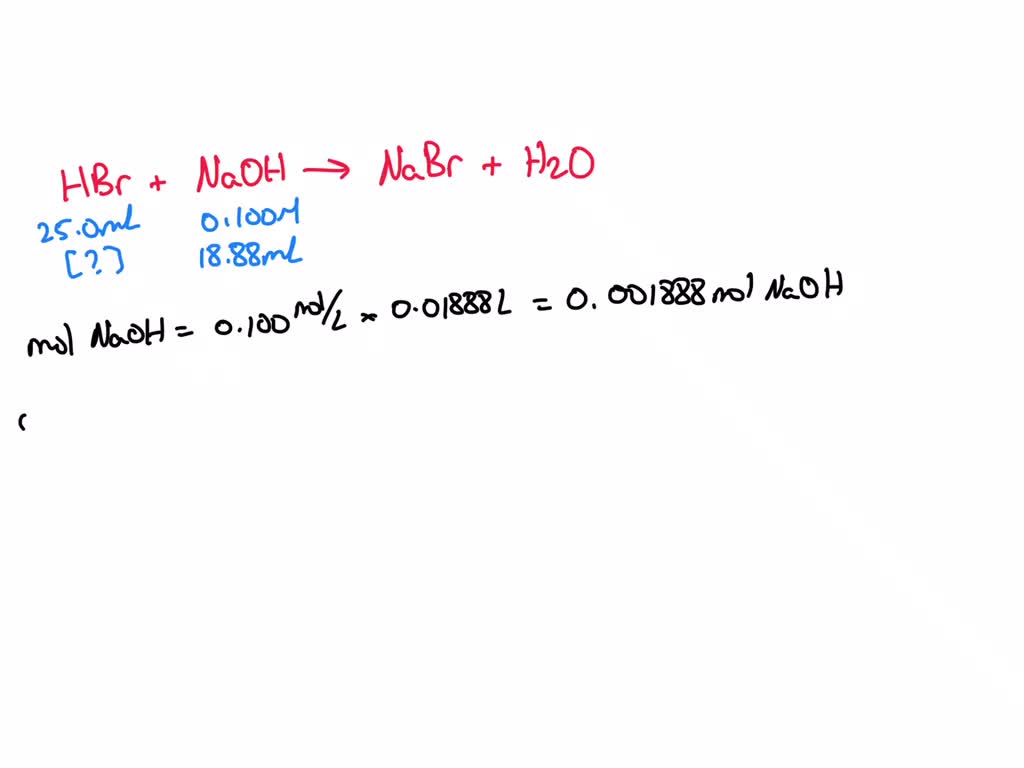
Source Image: numerade.com
Download Image
Consider the titration of a 25.0-mL sample of 0.175 M CH3NH2 with… | Channels for Pearson+ The four parts of the titration curve are described below and you should look to the approriate text section to see how they are treated. Pure Acid (0 ml of base is added, section 17.3.2.1) Excess acid (you have not added enough base to neutralize all of it and so have a buffer of the weak acid and it’s salt, section 17.3.2.2)

Source Image: pearson.com
Download Image
Answered: solution of 0.100 M HCl and a solution… | bartleby The titration of 80.0 mL of an unknown concentration H* 3PO4 * solution requires 126 mL of 0.218 M (molarity) KOH solution. What is the concentration of H* 3PO4 * solution (in M)? I go through all of the calculations and get 0.343 M, but apparently this is wrong. I’m pretty sure that I’ve set it up correctly. I’m lost here. Sort by:
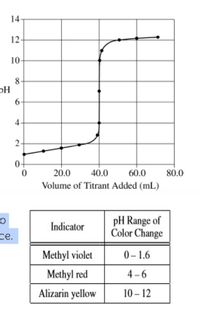
Source Image: bartleby.com
Download Image
Acid–Base Titrations A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH (the titration curve is shown in Figure 14.18). Calculate the pH at these volumes of added base solution: (a) 0.00 mL (b) 12.50 mL (c) 25.00 mL (d) 37.50 mL. Solution (a) Titrant volume = 0 mL. The solution pH is due to the acid ionization of
Source Image: saylordotorg.github.io
Download Image
Consider the titration of a 25.0-mL sample of 0.175 M CH3NH2 with… | Channels for Pearson+
Acid–Base Titrations To calculate the pH of the solution, we need to know [H+], which is determined using exactly the same method as in the acetic acid titration in Example 17.4.2: final volume of solution = 100.0mL + 55.0mL = 155.0mL. Thus the concentrations of Hox− and ox2− are as follows: [Hox−] = 3.60 mmolHox− 155.0 mL = 2.32 ×10−2 M.
SOLVED: A 25.0-mL sample of an unknown HBr solution is titrated with 0.100 M NaOH. The equivalence point is reached upon the addition of 18.88 mL of the base. What is the Answered: solution of 0.100 M HCl and a solution… | bartleby The four parts of the titration curve are described below and you should look to the approriate text section to see how they are treated. Pure Acid (0 ml of base is added, section 17.3.2.1) Excess acid (you have not added enough base to neutralize all of it and so have a buffer of the weak acid and it’s salt, section 17.3.2.2)