In general, to calculate the total energy of a given conformation, add all the torsional and steric strain: For example: Calculate the energy difference between these two conformations of butane: The first conformation has one CH 3 /CH 3 gauche interaction which brings 3.8 kJ/mol energy of destabilization. The second conformation is three pairs
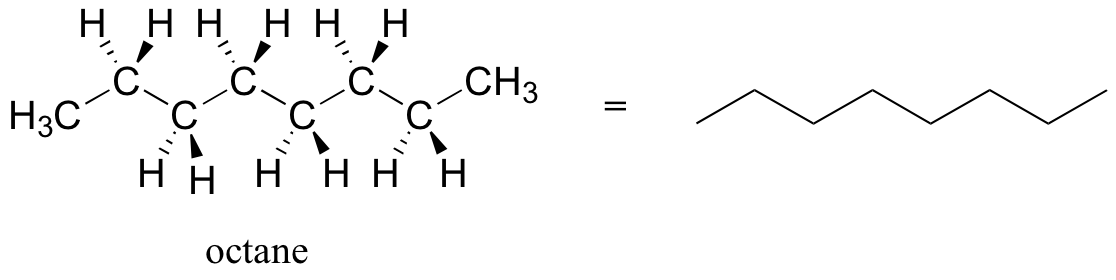
3.9: Conformations of Butane – Chemistry LibreTexts
A totally eclipsed conformation of butane would have a potential energy of ~21 kJ while the more stable eclipsed conformation would have a potential energy of 15 kJ. Compared to the staggered conformations this is quite a lot larger. … So this one right here is also a Gauche conformation. So hopefully you understand now that, you just have to
Source Image: coursehero.com
Download Image
The gauche interaction occurs in butane occurs when the two methyl groups have dihedral angles of 60° and 300° and arises because the methyl groups are still quite close together (about 3.1 Å, compare to 2.9 Å) for the syn – conformation. The strain energy of the gauche interaction is about 0.9 kcal/mol.
Source Image: chem.libretexts.org
Download Image
How many gauche conformations are possible n-butane? Steric hinderance is the strain that causes an increase in potential energy due to the repulsion between the electron clouds of 2 bulky substituents on carbons that are not neighboring . If u look at 0:56

Source Image: byjus.com
Download Image
Which Of The Following Is A Gauche Conformation For Butane
Steric hinderance is the strain that causes an increase in potential energy due to the repulsion between the electron clouds of 2 bulky substituents on carbons that are not neighboring . If u look at 0:56 No headers. Now let us consider butane, a slightly larger molecule. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C 2 and C 3.Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position.. This is the highest energy conformation for butane, due to what is called
is the most stable conformation of butane.
Butane Conformations. Now let’s consider butane, with its four-carbon chain. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C 2 and C 3.Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position, with the two C-C bonds at a 0 o dihedral angle. Conformational Analysis of Butane (Anti – Eclipsed – Gauche) | Read Chemistry

Source Image: readchemistry.com
Download Image
What is the difference between gauche and staggered conformations? – Quora Butane Conformations. Now let’s consider butane, with its four-carbon chain. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C 2 and C 3.Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position, with the two C-C bonds at a 0 o dihedral angle.
Source Image: quora.com
Download Image
3.9: Conformations of Butane – Chemistry LibreTexts In general, to calculate the total energy of a given conformation, add all the torsional and steric strain: For example: Calculate the energy difference between these two conformations of butane: The first conformation has one CH 3 /CH 3 gauche interaction which brings 3.8 kJ/mol energy of destabilization. The second conformation is three pairs

Source Image: chem.libretexts.org
Download Image
How many gauche conformations are possible n-butane? The gauche interaction occurs in butane occurs when the two methyl groups have dihedral angles of 60° and 300° and arises because the methyl groups are still quite close together (about 3.1 Å, compare to 2.9 Å) for the syn – conformation. The strain energy of the gauche interaction is about 0.9 kcal/mol.
Source Image: toppr.com
Download Image
Conformation of Butane, n-Butane, Conformation of n-Butane – YouTube Question: 20. Which of the following is a gauche conformation for butane? CH сн. нс сн, H H CH3 нүн Н -сна нен H -Н н CH3 H H н H HCHE OH — ІІ ІІ IV D. IV AI В. І С. ІІ Show transcribed image text Expert Answer 100% (2 ratings) Soluti … View the full answer Transcribed image text: 20. Which of the following is a gauche conformation for butane?
Source Image: youtube.com
Download Image
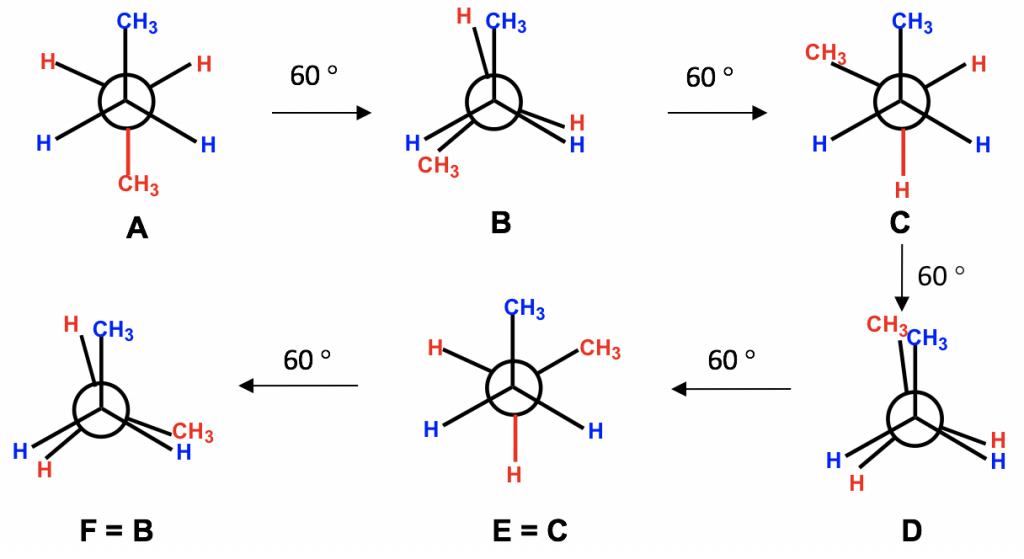
4.1 Conformation Analysis of Alkanes – Organic Chemistry I Steric hinderance is the strain that causes an increase in potential energy due to the repulsion between the electron clouds of 2 bulky substituents on carbons that are not neighboring . If u look at 0:56
Source Image: kpu.pressbooks.pub
Download Image
Conformational Analysis of Butane (Anti – Eclipsed – Gauche) | Read Chemistry No headers. Now let us consider butane, a slightly larger molecule. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C 2 and C 3.Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position.. This is the highest energy conformation for butane, due to what is called

Source Image: readchemistry.com
Download Image
What is the difference between gauche and staggered conformations? – Quora
Conformational Analysis of Butane (Anti – Eclipsed – Gauche) | Read Chemistry A totally eclipsed conformation of butane would have a potential energy of ~21 kJ while the more stable eclipsed conformation would have a potential energy of 15 kJ. Compared to the staggered conformations this is quite a lot larger. … So this one right here is also a Gauche conformation. So hopefully you understand now that, you just have to
How many gauche conformations are possible n-butane? 4.1 Conformation Analysis of Alkanes – Organic Chemistry I Question: 20. Which of the following is a gauche conformation for butane? CH сн. нс сн, H H CH3 нүн Н -сна нен H -Н н CH3 H H н H HCHE OH — ІІ ІІ IV D. IV AI В. І С. ІІ Show transcribed image text Expert Answer 100% (2 ratings) Soluti … View the full answer Transcribed image text: 20. Which of the following is a gauche conformation for butane?